Health Care Executives
Our scalable health care solutions align and integrate with your strategic goals to improve quality, accelerate decision-making, and propel progress across all service lines.
Services:
Clinicians
We are committed to empowering physicians, nurse practitioners, and physician assistants with the tools, technology, and support needed to deliver exceptional patient care.
- Nationwide opportunities
- Work-life balance
- Leading benefits
- Market-based compensation
- Remote care delivery
- Residency positions
- Career development
- Clinical technology tools
- Ongoing training and education
Join Our Team
A leader in clinical practice management, we work to align clinical and operational teams in order to deliver exceptional patient care and make a difference in the health care industry. Join our team and become a part of the revitalization of health care across the nation.
- Values-based environment
- Remote, hybrid, and in-person opportunities
- Company stability and growth
- Significant employee support
- Work-life balance
- Comprehensive benefits
- Market-based compensation
Clinical Services Results
Our commitment to continuous improvement in quality is evident in our results.

97% Clinician Retention Rate

99% MIPS
Scores
<1% Locums
Usage
15% Average Collections Increase
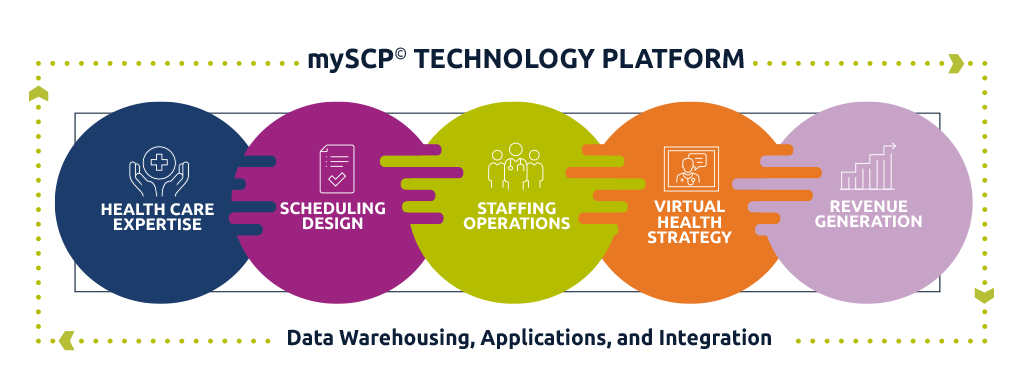
Health Care Services Experience

Capability Hubs
We structure systems for success, integrating technology, tools, and talent into a closed-loop process that amplifies evidence-based approaches, reinforces training and enablement, and ensures outcomes are achieved.
Local
- Recruit and retain local clinicians and medical directors and align with your strategic goals
Regional
- Provide regional operational and clinical leadership support, training, and guidance
National
- Invest in nationwide technology, resources, and capability development
Working with a
Health Care Partner
We pride ourselves in being a leader in the health care industry, always moving forward with innovative ideas to revitalize health care at every level. Our clinical and operational teams work together to sustainably extend the reach and increase the impact of health care across the nation.
Join Our Team4 Strategies to Transform the ICU by Bringing the C-Suite to the Bedside
While the ICU is a critical component of the hospital, it is often viewed as an expensive cost center. Erika Gabbard, DNP, RN, CCNS, CCRN-K explores how to break through siloed thinking and create a new, comprehensive approach and mindset to bring C-suite professionals to the bedside, achieving strategic needs of the hospital and aligning holistic cost with complex clinical management and the well-being of patients who require ICU-level care.
Latest White PaperInnovation and Technology

Innovation
Technology
Clinical Integration
Aligning Departments and Transforming Outcomes
With five decades of hospital practice management experience, our experts believe clinical integration delivers the best possible clinical outcomes and drives transformation in effectiveness, efficiency, and growth. An interdepartmental collaborative approach optimizes your financial spend and allows for cross-subsidization while delivering exceptional patient care.
0
+
states
0
+
health care facilities
0
M+
patients treated annually
0
+
talented clinicians

Annual Review 2022
SCP Health's 2022 Annual Review details our newly expanded portfolio, quality performance metrics and reports, and nation-wide health care advocacy opportunities.
Contact Us
Interested in a strategic health care partnership or career advancement by working with SCP Health? Please reach out by filling out the form to:
- Schedule a VIP meeting
- Request a consultation
- Speak with a clinical expert
- Chat with a recruiter
- Get in touch with a scheduler
- Contact a sales representative




 Care Delivery
Care Delivery Clinical Staffing
Clinical Staffing Clinical Workforce Optimization
Clinical Workforce Optimization Documentation & Revenue Cycle
Documentation & Revenue Cycle Virtual Health
Virtual Health Advanced Care in the Home
Advanced Care in the Home Clinical Integration
Clinical Integration